Medical Devices Regulation (MDR)
Regulation (EU) 2017/745 on medical devices (Medical Device Regulation – MDR) entered into force on 26.05.2021. It has replaced the Medical Device Directive 93/42/EEC (MDD) and also Directive 90/385/EEC (on active implantable medical devices - AIMDD).
The new regulation means that manufacturers of medical devices must meet stricter requirements in order to be able to sell their products in the EU. Among other things, the rules for conformity assessment procedures requiring the involvement of a notified body have been tightened. TUV NORD continues to be one of the European notified bodies qualified for these procedures, as was also the case under the old legislation (scope of designation TUV NORD CERT at NANDO).
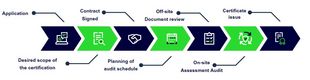
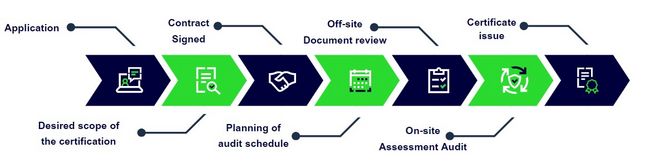
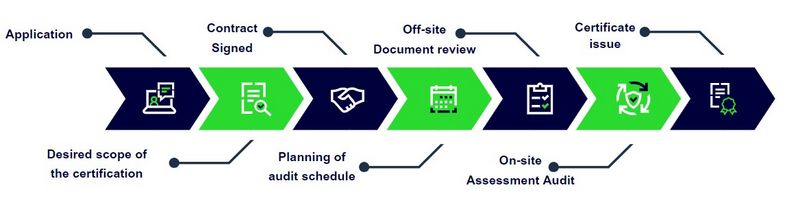
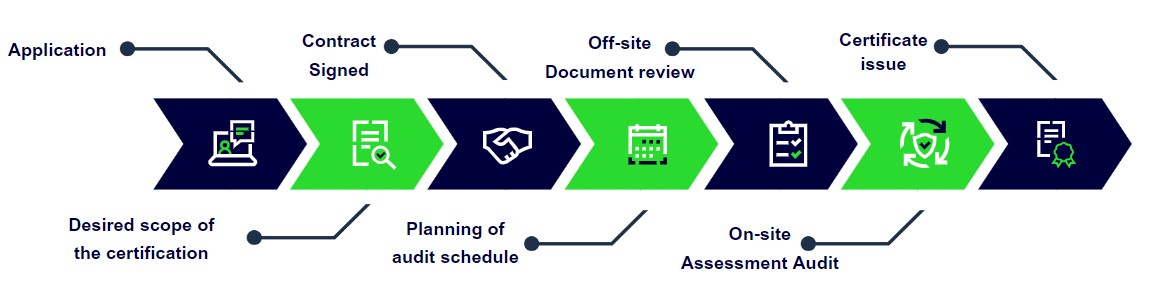
Certification Process